Published online by Cambridge University Press: 16 February 2011
Single-crystal Si films have been grown on Si(001)2×1 substrates by UVphotostimulated atomic-layer epitaxy (ALE) from Si2H6. The ALE deposition rate R per growth cycle remains constant at 0.4 monolayers (ML) over a wide range of deposition parameters: growth temperature (Ts= 180–400 °C), Si2H6 exposure (peak pressure during gas pulse = 0.1−5 mTorr), 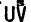 laser energy density (
laser energy density (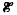 = 250–450 mJ cm−2 where
= 250–450 mJ cm−2 where 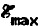 is determined by Ts), and number of UV laser pulses per cycle. A film growth mocrel, based upon the results of the present deposition experiments and Monte Carlo simulations, together with our previous adsorption/desorption measurements, Is used to describe the reaction pathway for the process. The Hterminated silylene-saturated surface formed by adsorption and desorption of disilene is thermally stable and passive to further Si2H6 exposure. ArF or KrF laser pulses (≅20 ns) are used to desorb H, following a Si2H6 exposure, and the growth cycle is repeated until the desired film thickness is obtained. At Ts < 180 °C, the growth process becomes rate limited by the surface dissociation step and R decreases exponentially as a function of 1/Ts with an activation energy of ≅0.5 eV. At Ts > 400 °C, H is thermally desorbed and pyrolytic growth competes with ALE. Transmission electron micrographs together with selected-area electron diffraction patterns show that the ALE films are epitaxial layers with no observed extended defects or strain.
is determined by Ts), and number of UV laser pulses per cycle. A film growth mocrel, based upon the results of the present deposition experiments and Monte Carlo simulations, together with our previous adsorption/desorption measurements, Is used to describe the reaction pathway for the process. The Hterminated silylene-saturated surface formed by adsorption and desorption of disilene is thermally stable and passive to further Si2H6 exposure. ArF or KrF laser pulses (≅20 ns) are used to desorb H, following a Si2H6 exposure, and the growth cycle is repeated until the desired film thickness is obtained. At Ts < 180 °C, the growth process becomes rate limited by the surface dissociation step and R decreases exponentially as a function of 1/Ts with an activation energy of ≅0.5 eV. At Ts > 400 °C, H is thermally desorbed and pyrolytic growth competes with ALE. Transmission electron micrographs together with selected-area electron diffraction patterns show that the ALE films are epitaxial layers with no observed extended defects or strain.