Introduction
The earliest known records of the dipteran family Asilidae are reported from the Lower Cretaceous Crato Formation in Brazil (e.g., Grimaldi Reference Grimaldi1990; Lamas et al. Reference Lamas, Sampronha and Ribeiro2021), although most asilid fossils are reported from Eocene, Oligocene, and Miocene deposits (Evenhuis Reference Evenhuis2017; Nel and Jouault Reference Nel and Jouault2023; Camargo and Gomes Reference Camargo and Gomes2024). Asilids are rare or uncommon in Cretaceous deposits (Dikow and Grimaldi Reference Dikow and Grimaldi2014), and fossils have yet to be reported from the Quaternary. The reasons for this are likely due to (1) taphonomy and (2) sampling bias.
Taphonomy, the study of the time and processes between the death of an organism and its recovery as a (sub)fossil, should not be underestimated when interpreting any fossil deposit, and for the present study, Quaternary entomological remains. Most subfossil assemblages from Quaternary-aged sediments are dominated by Coleoptera because of their heavily sclerotised exoskeletons, which are more likely to preserve, and their predominance in most habitats (e.g., Miller et al. Reference Miller, Voss-Foucart, Toussaint and Jeuniaux1993; Bouchard et al. Reference Bouchard, Smith, Douglas, Gimmel, Brunke, Kanda, Foottit and Adler2017). Asilids are comparably less robust, and their absence in most Quaternary deposits may simply be a result of the lack of preservation rather than of their absence from the landscape.
Sampling procedures incorporate a level of bias that may preferentially recover the remains of certain fauna. The sampling of rodent middens, as in the present study, has proven to be an exceptional resource for botanical, invertebrate, and vertebrate remains (Van Devender and Mead Reference Van Devender and Mead1978; Zazula et al. Reference Zazula, Froese, Westgate, La Farge and Mathewes2005, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2011; Cocker et al. Reference Cocker, Zazula, Hall, Jass, Storer and Froese2024) but can be subject to biases such as selective caching (Gillis et al. Reference Gillis, Morrison, Zazula and Hik2005; Zazula et al. Reference Zazula, Mathewes and Harestad2006). Again, the omission of taxa from Quaternary entomological records is not evidence of their absence and instead may represent sampling biases outside the control of palaeoecological studies.
This is not to say that flies are not represented in Quaternary literature. Members of Chironomidae have routinely been used to study palaeoclimates (Brooks Reference Brooks2006), lake eutrophication and acidification, and lake productivity (Brodersen and Quinlan Reference Brodersen and Quinlan2006) as a function of their aquatic larval stages. However, the omission of asilid records in Quaternary deposits stands in contrast with their nearly ubiquitous modern distribution. This is likely in part due to their terrestrial life cycle and often xerophilic habitat, leading to a lower chance of preservation in common fossil-bearing deposits such as aquatic sediments or amber (Dikow and Grimaldi Reference Dikow and Grimaldi2014). In the present study, we report the first Quaternary record of an asilid fly in the genus Lasiopogon Loew (Diptera: Asilidae) recovered from an Arctic ground squirrel (Rodentia: Sciuridae) midden, about 16 500 years old, preserved in permafrost in central Yukon Territory, Canada.
During much of the Pleistocene Epoch, spanning approximately 2.5 million to 11 000 years before present, almost all of Canada was covered in ice. During this time, central Yukon Territory, Canada, and Alaska, United States of America, were ice-free and were linked to eastern Siberia by the Bering Land Bridge, a stretch of land as broad as present-day Alaska. This expanse of tundra and grassy steppes is known to geographers and biologists as Beringia (Matthews Reference Matthews1979; Schweger Reference Schweger, Danks and Downes1997).
Lasiopogon (Diptera: Asilidae)
Lasiopogon species are relatively setose asilids that average 8–10 mm in length. Typically, they are black with patterns of brown or grey tomentum (Fig. 1). Lasiopogon adults are opportunistic predators of small insects and are usually found on the ground or on logs and rocks. The larvae are also predaceous and occur in a variety of habitats, often near water, that include sandy soils, subalpine meadows, dry forests, and heaths (Cannings Reference Cannings2002; McKnight and Cannings Reference McKnight and Cannings2020). Extant Lasiopogon species occur in temperate regions across the Northern Hemisphere. Some species have more northerly ranges than any other asilids: individuals have been collected in habitats bordering the Arctic Ocean in North America and along the northern sections of the major Siberian rivers (Cannings Reference Cannings2002). This Holarctic genus has around 100 species organised into about 16 species groups, but the phylogenetic relationships within and among many of these groups are still being resolved, and groups for many European species are still undefined (Cannings Reference Cannings2002; McKnight and Cannings Reference McKnight and Cannings2020). Five species are found in the North American side of Beringia, each from a different species group: L. hinei Cole and Wilcox, L. prima Adisoemarto, L. canus Cole and Wilcox, L. yukonensis Cole and Wilcox, and L. cinereus Cole.

Figure 1. Lasiopogon canus male, Whitehorse, Yukon, Canada, 27 June 2022. Photo by Jukka Jantunen (iNaturalist 126443592).
Most species of Lasiopogon now living in eastern Asia probably evolved from populations in eastern North America around the mid-Miocene (∼15 million years ago; Cannings Reference Cannings2002). Two Beringian species – L. hinei and L. prima – are closely related to Eurasian flies (Cannings Reference Cannings, Danks and Downes1997). These taxa, or their ancestors, likely returned to North America across the Bering Land Bridge. Both L. hinei and L. prima are found in dune habitats (Fig. 2), on south-facing grassland slopes (Fig. 3), in dry forest habitats, and along sandy streams in the Yukon. Lasiopogon hinei ranges across Eurasia, from northwest Russia eastwards into North America, and would have existed in Beringia when Eurasia and North America were connected via the Bering Land Bridge. A portion of the population would have been isolated in North America during the last interglacial period when the Bering Land Bridge was flooded, and the melting of continental ice masses would have allowed the species to expand southwards into British Columbia and Alberta, Canada. All other members of the hinei species group are found only in eastern Asia (Cannings Reference Cannings2002). Lasiopogon prima is in a similar situation, being most closely related to two Asian species – L. septentrionalis Lehr and an undescribed Mongolian species (McKnight and Cannings Reference McKnight and Cannings2020) – and only distantly related to the Nearctic taxa of its terricola species group. Following the deglaciation at the end of the Pleistocene, L. prima extended its range from northern British Columbia to southeast Alberta and the Athabasca dunes of northern Saskatchewan, Canada. In Alaska and the Yukon Territory, L. prima populations are found along river valleys all the way to the Arctic coast. Two other Beringian species – L. canus and L. yukonensis – are in species groups (respectively, the canus and aldrichii groups) whose recent radiations are exclusively North American. Nevertheless, they belong to a basal cinctus clade that is otherwise mostly Palaearctic (McKnight and Cannings Reference McKnight and Cannings2020). It is unclear when they separated from their Asian relatives and whether this was a Beringian dynamic. The most common of the Beringian Lasiopogon species, L. canus, is recorded from Alaska, Yukon Territory, and the northwestern Northwest Territories, Canada. This distribution is consistent for a species that existed in a refugium in eastern Beringia during the last glacial period and subsequently dispersed short distances following deglaciation. A final species, L. cinereus, is rarely observed in the Yukon Territory; its origins lie to the south along with all other species of its group, the opaculus section (Cannings Reference Cannings, Danks and Downes1997, Reference Cannings2002).

Figure 2. Dunes at Carcross, Yukon Territory, Canada (60.1083°, –134.7370°). Lasiopogon canus, L. hinei, L. prima, and L. yukonensis have all been collected here. Photo by Syd Cannings.

Figure 3. South-facing grassland slopes at Stepping Stone, Pelly River, Yukon Territory (62.7996°, –137.3271°), a common habitat of Lasiopogon canus and L. hinei. The yellow flower is Yukon Goldenweed, Nestotus macleanii (Brandegee) R.P. Roberts et al. (Asteraceae), a Yukon endemic. Photo by Syd Cannings.
Materials and methods
Site and midden description
The Lucky Lady II site (63.729°, –139.121°) is located approximately 46 km south of Dawson City in the Klondike goldfields of central Yukon Territory and on the traditional territories of the Tr’ondëk Hwëchin First Nation (Fig. 4). Placer gold mining has exposed a rich Late Pleistocene succession that has been studied for several years (Monteath et al. Reference Monteath, Kuzumina, Mahoney, Calmels, Porter and Mathewes2023).
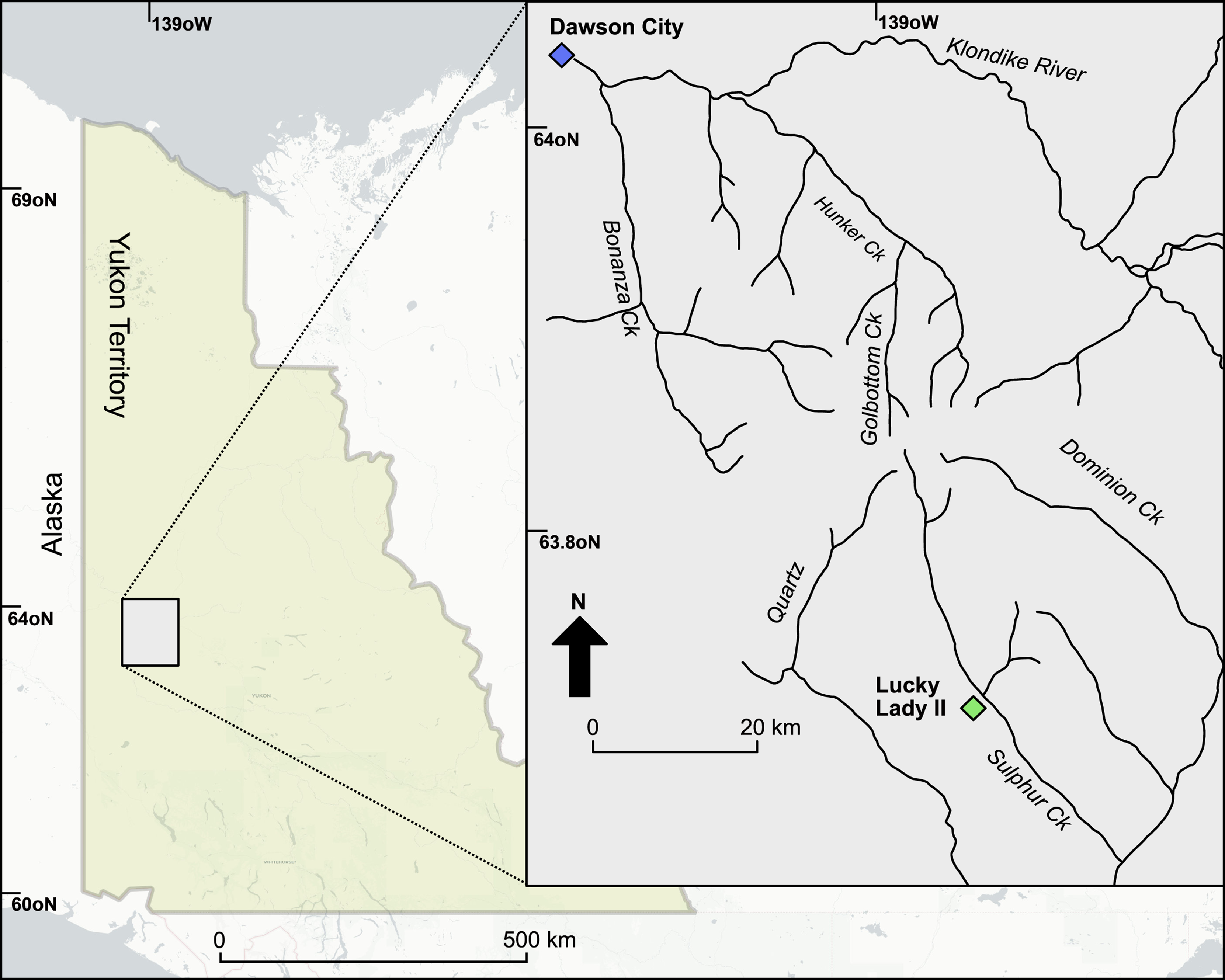
Figure 4. Map showing location of Lucky Lady II site, Klondike Valley, Yukon Territory, Canada.
The Lucky Lady II site has recently been the focus of an interdisciplinary study by Monteath et al. (Reference Monteath, Kuzumina, Mahoney, Calmels, Porter and Mathewes2023) that presents the best-documented Pleistocene–Holocene transition in the region. As is common within Quaternary entomological studies, the authors recovered almost entirely coleopteran remains except for some hymenopterans in their Holocene-aged sediments. An Arctic ground squirrel, Urocitellus parryii Richardson (Rodentia: Sciuridae), midden, sample BJ11-LLII-63, was recovered in 2011 (Fig. 5) and radiocarbon dated to 13 665 ± 35 14C years or approximately 16 510 cal year BP. (The latter age represents a median calibrated version; see Table 1.) The midden is approximately 30 cm in diameter and comprises mostly grassy nesting material used to line the hibernaculum. The midden also contains a significant seed cache that is the subject of another study by S.C. The subfossil asilid fly reported in this study was collected directly from within this seed cache.

Figure 5. Arctic ground squirrel midden, Lucky Lady II site, Klondike goldfields, central Yukon Territory, Canada. Photo by Britta Jensen.
Table 1. Radiocarbon and calibrated ages for Arctic ground squirrel midden, BJ11-LLII-63. The calibrated age is presented at 2σ uncertainty (95.4% confidence interval)

Chronology
We prepared one sample from the seed cache (a capsule of Phlox cf. hoodii Richardson (Polemoniaceae)) for radiocarbon dating by completing pretreatment at the University of Alberta (Edmonton, Alberta, Canada) using an acid–base–acid methodology (e.g., Reyes et al. Reference Reyes, Jensen, Zazula, Ager, Kuzmina, La Farge and Froese2010). The sample was subsequently frozen, freeze-dried, and stored in airtight sterilised vials. We then shipped the sample to the W.M. Keck Carbon Cycle Accelerator Mass Spectrometer facility at the University of California, Irvine (UCIAMS; Irvine, California, United States of America), where CO2 production, graphitisation, and measurement of radiocarbon abundance were completed. We calibrated the single radiocarbon date (Table 1) using OxCal, version 4.4 (Bronk Ramsey Reference Bronk Ramsay2009) and the IntCal20 calibration curve (Reimer et al. Reference Reimer, Austin, Bard, Bayliss, Blackwell and Bronk Ramsey2020).
Photographs of the subfossil head were taken with a Nikon Z5 camera (Nikon, Minato City, Tokyo, Japan), fitted with a Canon MP-E 65MM macro lens (Canon, Ota City, Tokyo, Japan), and focus stacks were made with a WeMacro focus stacking rail (Shanghai Macro Photoelectric Technology Co., Ltd., Shanghai, China). Images were stacked in Helicon Focus 8 (Helicon Soft Limited, Kharkiv, Ukraine). Final edits were made in Adobe Photoshop (Adobe, San Jose, California, United States of America).
The subfossil Lasiopogon head is accessioned in the Government of Yukon’s Yukon Palaeontology collections in Whitehorse, Yukon Territory (https://yukon.ca/en/access-fossil-collection).
Description and identification of the subfossil
The subfossil (Fig. 6) consists of one head capsule that is mostly intact, with small damage holes in each compound eye on the ventral side where mouthparts had attached (now missing) and at the occipital foramen (where the neck had attached). The antennae and most setae are also missing, but sockets remain that allow us to infer their position.

Figure 6. Subfossil head of Lasiopogon sp.: A., anterior view; B, dorsal view; C, lateral view; D, posterior view.
The sunken vertex, macrosetae sockets indicating an extensive mystax, dichoptic eyes, and gestalt are characteristic of Asilidae. The specimen is identifiable as belonging to the subfamily Stichopogoninae, based on the combination of two morphological apomorphies (Dikow Reference Dikow2009): the posterior margin of the compound eyes is distinctly sinuate in the ventral half (visible in lateral view), and the frons abruptly diverges laterally beginning at the antennal insertion (visible in anterior view). The facial swelling is well developed, extending from the mouth nearly to the antennal bases, and sockets show that the mystax extended over most of the face. The combination of these two characters excludes other genera from the subfamily and are indicative of Lasiopogon (Fig. 7; Cannings Reference Cannings2002; McKnight and Cannings Reference McKnight and Cannings2017). The head cannot be identified further than genus at this time: characters to separate species typically require additional body parts, and DNA barcoding was not attempted. Insect chitin is still a challenging material for ancient DNA extraction, and although methodologies are rapidly advancing in this field (Thomsen et al. Reference Thomsen, Elias, Gilbert, Haile, Munch and Kuzmina2009; Smith et al. Reference Smith, Kamiński, Kanda, Sweet, Betancourt and Holmgren2021), we await further progress before risking this unique specimen.

Figure 7. Head of Lasiopogon canus, female, Royal British Columbia Museum specimen ENT991-86275. Old Crow, Yukon Territory, Canada, 6 July 1983, collected by Robert A. Cannings: A, anterior view; B, lateral view.
Beringia and Lasiopogon palaeoecology
As previously discussed, Lasiopogon species in the present-day Yukon Territory are typically found in dune habitats, on south-facing grassland slopes, in dry forest habitats, or along sandy streamsides (Cannings Reference Cannings, Danks and Downes1997). Of particular interest are the species living on south-facing slopes, given the habitat similarity between these relict grasslands and the now-extinct steppe–tundra (“mammoth steppe”) ecosystem that was dominant during the Pleistocene in this region. Numerous studies have contributed to our understanding of Pleistocene steppe–tundra ecosystems for which the consensus of a grass- and forb-dominated landscape is widely accepted but without a modern analogue (Guthrie Reference Guthrie2001; Murchie et al. Reference Murchie, Monteath, Mahony, Long, Cocker and Sadoway2021). Arctic ground squirrel middens are important sources of data for reconstructions of Beringian environments (Zazula et al. Reference Zazula, Froese, Westgate, La Farge and Mathewes2005, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2011; Lopatina and Zanina Reference Lopatina and Zanina2006; Zanina et al. Reference Zanina, Gubin, Kuzmina, Maximovich and Lopatina2011). Seminal research by Zazula established the role of Arctic ground squirrel middens as valuable archives of plant macrofossils spanning the last 80 000 years in the Yukon Territory (Zazula et al. Reference Zazula, Froese, Westgate, La Farge and Mathewes2005, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2011). When combined with the preservation potential of permafrost, much of the material is so exquisitely preserved that identifications can be made with increased taxonomic resolution when compared to other biological proxies, such as pollen. This local record of Beringian ecosystems provides a unique perspective of steppe–tundra diversity throughout the Late Pleistocene.
The plant macrofossil assemblage from the midden (BJ11-LLII-63) generally aligns with the interpretation of a cold and dry landscape dominated by low-lying vegetation (S.C., unpublished data), similar to the south-facing slopes in the Yukon today (Fig. 3). The midden is dominated by capsules of the forb, Phlox cf. hoodii, which has been reported to be a subdominant taxon on grassland slopes of the Aishihik–Sekulmun Lakes area of the Yukon Territory and a dominant taxon across grasslands of the northern Great Plains (Vetter Reference Vetter2000). These regions are consistent with the habitats of some Lasiopogon species and similarly provide an appropriate interpretation of the palaeoecological data recovered from midden BJ11-LLII-63.
Given the lack of Quaternary data for asilid flies and for the genus Lasiopogon, the data can conservatively suggest habitat continuity in this genus for at least the last 16 500 years. However, given the considerable reduction in available habitat following the collapse of steppe–tundra ecosystems in Beringia associated with the Pleistocene–Holocene transition, it is interesting to postulate whether Lasiopogon may have been more or less widespread during the Pleistocene. Such a decline in population is recorded by other invertebrate taxa, specifically the Yukon endemic weevil, Connatichela artemisiae Anderson (Coleoptera: Curculionidae) (Anderson Reference Anderson1984). Quaternary entomological data from the region suggests a considerable collapse in C. artemisiae populations associated with a decline in prairie sage, Artemisia frigida Willdenow (Asteraceae). These two taxa are linked: the larvae of C. artemisiae feed on the roots of A. frigida, and adults often use the plant during copulation (Anderson Reference Anderson1984; Monteath et al. Reference Monteath, Kuzumina, Mahoney, Calmels, Porter and Mathewes2023). If the Pleistocene habitat was more fitting for Lasiopogon than modern conditions are and regional species were more common, that could help explain how the patchy distributions common to this group were first established. On the other hand, the Pleistocene habitat could have been submarginal, and Lasiopogon may have been merely one of the few asilids that could colonise parts of it. Lasiopogon spp. are one of the more cold-adapted genera of robber flies and are usually one of the earliest to emerge in the spring (Cannings Reference Cannings2002; McKnight and Cannings Reference McKnight and Cannings2020).
Biogeographically, the recovery of this Lasiopogon head presents compelling evidence to establish the presence of the genus at a time and place where Beringian exchange has been presumed. Probable Beringian splits are found at several levels of the Lasiopogon phylogeny – for example, populations within a species (e.g., L. hinei), species within a group (e.g., L. prima and L. septentrionalis), and species groups along the backbone (e.g., the canus group in the cinctus clade). As more evidence such as the discovery reported here comes to light, future phylogenetic and biogeographic work seeking to model likely histories that explain these patterns and improve our understanding of the impressive adaptive radiation of this genus may be possible.
Conclusion
We have presented the first Quaternary record of an asilid fly, Lasiopogon sp. (Diptera: Asilidae), from North America. The preservation of this asilid head is best attributed to the preservation potential of permafrost and, more significantly, to the unique taphonomic setting provided by the middens of Arctic ground squirrels that facilitates preservation in dry terrestrial environments.
This record provides a unique opportunity to understand the long-term ecology and history of Lasiopogon populations in Canada’s Yukon Territory, particularly when considering the ecosystems that developed following the last glacial maximum.
Acknowledgements
The authors thank Syd Cannings for the use of his habitat photographs (Figs. 2 and 3), Jukka Jantunen for the image of Lasiopogon canus (Fig. 1), Britta Jensen for the image of the Arctic ground squirrel midden (Fig. 5), and Hugh McIntosh (Royal British Columbia Museum, Victoria, British Columbia, Canada) for taking the photographs of the subfossil (Fig. 6) and museum specimen (Fig. 7). Syd Cannings commented on a draft of the paper. This research was funded by University of Alberta Northern Research Award Grants to S.L. Cocker. We thank two anonymous reviewers for their comments that greatly improved the manuscript.
Competing interests
The authors declare they have no competing interests.